Nanostars guide the way to better disease treatment and diagnosis
Rizia Bardhan, associate professor of chemical and biological engineering and Iowa State University Nanovaccine Institute researcher, is making life-saving advances in disease diagnosis and treatment by integrating nanoparticles and Raman spectroscopy.
Predicting immunotherapy success
Immunotherapy is a groundbreaking new treatment for cancer and other immune system related diseases. But, right now only 20–25 percent of patients respond to immunotherapies – and there’s no way to predict response or evaluate treatment effectiveness.
In a project supported by the National Institutes of Health, Bardhan uses an image-guided approach to solve this challenge by designing immunoactive gold nanostars. The gold nanostars will combine two medical imaging techniques, Positron Emission Tomography (PET) and Raman spectroscopy, which are widely used in guiding clinical therapies.
These nanostars will simultaneously detect both tumor cells expressing PD-L1 biomarker and also immune cytotoxic CD8 T cells directly in real time in vivo in animal models engrafted with breast cancer patient tumors.
“By tracking both cell types, our approach will help oncologists determine which patients are good candidates for immunotherapies and distinguish those who will not respond even before the start of treatment,” said Bardhan.
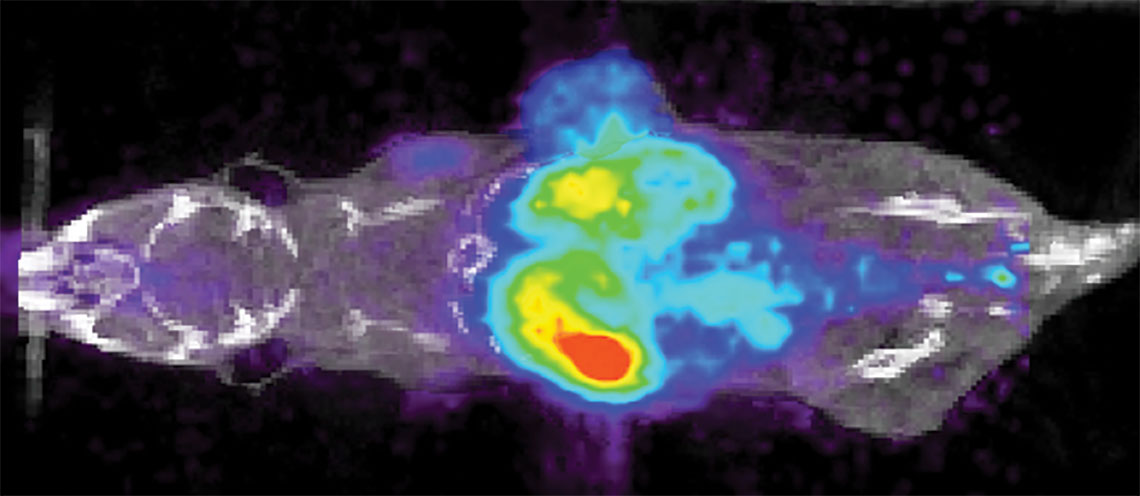
Tailored colorectal cancer treatment
Leveraging the strengths of Raman spectroscopy as a cancer diagnostic, Bardhan leads a Department of Defense-funded project to predict how colorectal cancer patients will respond to molecular therapies.
Metastatic colorectal cancer is highly lethal with limited treatment options, so rapid, accurate diagnostic tools are key to getting patients the most effective treatment at the earliest time point possible.
But how to determine what is the best treatment for each patient? Bardhan’s answer is using “organoids,” 3D culture systems that closely mimic the human tumor microenvironment.
“Drug discovery studies often use the ‘one mouse, one patient’ paradigm, where each mouse is engrafted with an individual patient tumor. But that approach is too expensive, slow and low-throughput for effective, timely clinical decisions,” said Bardhan.
Her team will instead derive more than 100 organoids from each patient tumor and treat them with a panel of drugs, some of which are both standards of care in colorectal cancer patients and others in clinical trials.
They will then perform high-throughput drug screening in these organoids with Raman spectroscopy and apply machine learning to distinguish patients that will respond to drugs. Their approach will both enable individualized treatment planning for patients and increase the efficiency of drug efficacy studies.
Bardhan is collaborating with Jonathan Mochel, associate professor of biomedical sciences at Iowa State’s College of Veterinary Medicine; Soumik Sarkar, associate professor of mechanical engineering; and others.
Early, accurate, affordable preterm labor screening
Bardhan’s expertise in Raman spectroscopy and nanoparticles extends far beyond cancer. She also leads a National Institutes of Health project to develop a cutting-edge new technology in her lab to detect biomarkers that lead to spontaneous preterm labor.
Preterm labor occurring before 37 weeks gestation results in more than 1 million childhood deaths globally, but current clinical methods to identify high risk of preterm labor have not been successful in reducing neonatal deaths. Bardhan’s approach will address this ongoing global clinical challenge.
Bardhan’s PRADA diagnostic platform – or portable reusable accurate diagnoses with nanostar antennas – consists of the same gold nanostars Bardhan’s team uses in other research efforts, but they are now labeled with targeting agents, such as Raman molecules, to allow multiplexed detection of clinically relevant biomarkers with high sensitivity and specificity. PRADA is also reusable, allowing more than 15 uses of the same sensor chip, reducing the overall cost.
Bardhan has teamed up with obstetrics and gynecology experts to generate a unique “PRADA maternal risk score” by studying patient serum both at risk of preterm labor and those with normal pregnancy. PRADA can ultimately enable early, accurate and affordable bedside screening for pregnant patients, a significant advance compared to current clinical measures.
